We spend a lot of time here talking about green energy and the need to divert our energy consumption from nonrenewable sources to more renewable solutions. We’ve gone over popular energy sources like solar and wind power, and figured out how they turn seemingly invisible energy into electricity that can power our homes, power our cars and power our apartment buildings.
When we look into these renewable resources, however, one major barrier sustainability engineers face is that, unlike gasoline, sunlight and wind cannot be generated on-demand. It’s hard for clean electricity to be stored in large quantities for use as needed in, say, a car, unlike a gasoline-powered car that can store the gasoline neatly inside until use.
The electricity that solar and wind power generates can run directly through power lines to homes, businesses and wherever else they are needed, but when there’s an excess of power generated, then that electricity has nowhere to go. There’s also no way to generate that electricity if the wind isn't blowing or the sun isn't shining, so finding a way to store that clean electricity for use later on would alleviate the need to rely on fossil fuels in the interim.
Essentially, fuel cells are a form of battery pack that can be used to store energy created by the electricity generated by renewable sources. That’s important to note, by the way, because the electricity itself isn't there already, but it does contain energy that can make electricity… if that makes sense. We’ll go into it more in a bit.
Let’s take a look at hydrogen fuel cells which are the most popular of fuel cells in the sustainability field.
How do hydrogen fuel cells work?
A hydrogen fuel cell starts with water, of all things. Water and electricity, actually.
Water is made of two hydrogen molecules and one oxygen molecule, with its chemical composition written down as H2O. When electricity is added to water, the electrically-charged current splits the oxygen molecules from the hydrogen ones, creating pure hydrogen gas and impure oxygen gas. In fact, you can test this process of electrolysis right at home with a glass of water, a battery and some paper clips.
Once separated from the oxygen, the hydrogen gas can be stored until future use in hydrogen fuel cells.
Now, the pretty cool thing about fuel cells is that they essentially reverse the process of electrolysis and produce electricity as a result. The cells intake hydrogen, remove the energy and add oxygen to create, you got it, water! And all that energy they removed? That goes to power your car, your phone, a lightbulb or whatever you’re using a fuel cell for, and the only emission byproduct is plain ol’ water, air and heat.
Pretty freaking nifty, if we do say so ourselves.
To boil it down a little more, we’ve got this handy diagram that shows how fairly simple this process is.
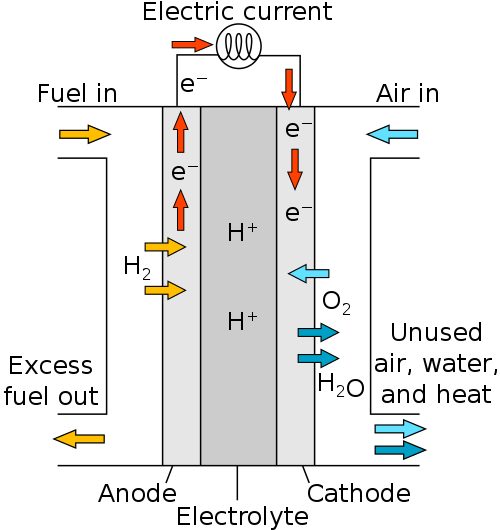
Hydrogen gas (H2) enters the first of a three-part chamber separated by negative and positive electrodes. As the gas passes through the negative electrode into the membrane between the electrodes, the hydrogen is split into electrons and protons. The electrons travel along the negative electrode to the positive electrode as an electric current, powering whatever is connected in between. Meanwhile the positively-powered protons travel through the membrane to hang out, play cards, grab a coffee or whatever it is protons do for fun these days.
The electric current continues to the positive electrode, where they attract and join again with the hydrogen protons in the membrane. Oxygen is pumped into the third chamber and interacts with the hydrogen gas to form, you guessed it, water!
The water is pumped out of the fuel cell in order to allow more space for more fuel, and the process just continues until there is no more hydrogen gas to input.
Benefits of hydrogen fuel cells
Hydrogen fuel cells offer a clean and efficient energy source that, if powered with cleanly-generated electricity, can greatly reduce emissions during the energy gathering process.
For one, hydrogen is one of the most abundant elements in the universe and is easily and readily accessible in our atmosphere and water sources. It’s nontoxic and clean to work with, and if it’s manufactured using renewable energy sources like wind and solar, then there are very few greenhouse gasses or emissions released in its production and use.
Another benefit is that hydrogen-powered fuel cells can be extremely efficient, with anywhere from 35-70% of the total energy generated in the cell’s reactions able to be used. Some cells can also utilize the excess heat byproduct in boosting its power, bringing the total efficiency up to around 90%!
Uses for hydrogen fuel cells
Although not as popular as battery-powered electric vehicles, hydrogen electric vehicles are starting to make more of an appearance in major countries such as Great Britain, China, Italy and Japan. As of 2022, Germany is the world’s leader in terms of implementing hydrogen fuel cell technology in city infrastructure, utilizing the cells in its buses and trains in an effort to produce clean energy on a scale as yet unheard of.
The only drawback to this green technology is that it does require a massive infrastructure overhaul to support a city run entirely on sustainable power. Thankfully, though, this is a problem we can solve and one we can continue to work on throughout our lifetimes!
So, next time you see some battery-powered buses or hear about hydrogen fuel cell technology, you’ll have a better idea as to what sustainability engineers are developing for the future of clean energy!
Pin it!

Featured photo courtesy Pixabay/trinspotterflo
Graphic courtesy Wikimedia Commons/Mattuci


 View All Posts by Colleen Ford
View All Posts by Colleen Ford